Overview
The Department of Pharmaceutical Quality Assurance, established in 2002 in MCOPS, fosters research and education for undergraduate, postgraduate, and research students.? The department offers a master's program in Pharmaceutical Quality assurance with an intake of 15 students.
The department is equipped with state-of-art laboratories, ultra-modern facilities, and competent staff, which have been considered as the most sophisticated infrastructure in the country. The Department of Pharmaceutical Quality Assurance also has access to licensed e-CTD software (Take solutions) to train the postgraduate students in drug e-filing procedures. The department also offers opportunities for research students seeking Ph.D. degrees. The focused research areas of the department are
i) Drug Metabolism and Pharmacokinetic studies,
ii) Development of analytical and bioanalytical method and validation,
iii) Pharmaceutical materials science including crystal engineering and crystal structure manipulations of drug molecules
iv) Quality management system of the pharmaceutical industry
v) Expertise in preparation and submission of dossiers.
The department undertakes research projects from the government as well as private funding agencies. A number of research articles are published in reputed journals every year. The department has marked its importance in research activities and also it has an excellent placement record. Academic and research excellence is achieved by dynamic curriculum, syllabus, and pedagogy to meet the current requirements of pharmaceutical industries, academics, and regulatory bodies.
Key Features
- State-of-the-art facility.
- Diversified capabilities.
- Team of academicians with rich industry expertise and international exposure.
- Specialized and focused on analytical services.
- Patient sample analysis for therapy planning and outcome.
Core competencies
- Drug Metabolism and Pharmacokinetic studies.
- Pharmaceutical materials science including Crystal engineering and crystal structure manipulations of drug molecules.
- Stability studies of drug substances and formulations.
- Analytical and bio-analytical method development and validation.
- Quality management of the pharmaceutical industry.
- Expertise in preparation and submission of dossiers.
?
PROGRAM
The program MPharm Pharmaceutical Quality Assurance has been designed to meet the needs of the ever-increasing demands of the pharmaceutical industry, research organizations, and academic institutions with special emphasis on the quality management system and regulatory submissions.
Objective:
To prepare the students to be competent, honest, industry-ready quality professionals through value-added rigorous training.
Scope:
The potential syllabus empowers students to excel and provides a lot of scope in getting the placement in reputed companies/institutes. Every year 100% placement happens through Campus recruitments for both branches. The alumni of both programs are placed in reputed pharma companies, research laboratories, and academic institutions worldwide.
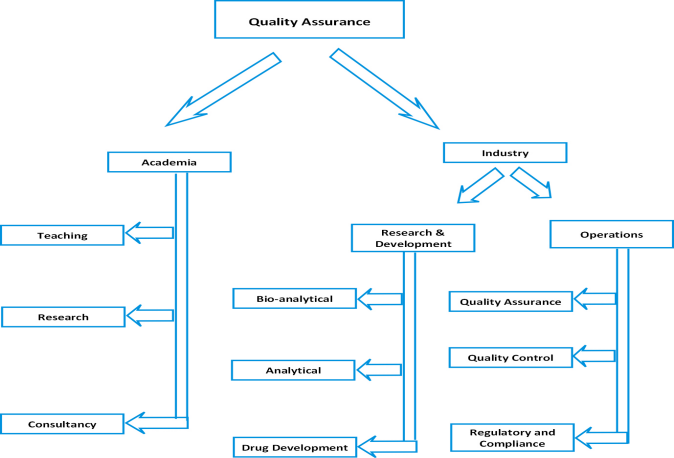
- Pharmaceutical Industries: There are different areas such as quality assurance/control, technology transfer, analytical and bioanalytical method development, production/ manufacturing, validation aspects, drug metabolism and pharmacokinetics (DMPK), clinical research organizations, intellectual property rights, pharmacovigilance, scientific writing and regulatory affairs where candidates of both the disciplines are getting recruited. The scope also includes Regulatory knowledge management, which is a greenfield service domain
- Entrepreneurship and consultancy: Postgraduates are also trained and encouraged to build their career as entrepreneurs to start pharmaceutical companies or as consultants to pharma companies
- Higher studies and academics: One can also go for higher degrees in research as well as for teaching jobs in academic institutions.
?
MPharm Pharmaceutical Quality Assurance
Course Work:
The course is designed to address the needs of the industry along with a keen emphasis on the scientific advancement of the field. It includes various aspects of Quality Assurance and related documentation, regulatory affairs, training, and analysis on advanced equipment. The course of study shall extend over a period of 4 semesters.
Program Educational Objectives (PEOs)
Click to view?Regulations for Mpharm Pharmaceutical Quality Assurance?programme
The course work for semesters includes the following subjects:
Semester I
PQA-MQA-101T: Modern Pharmaceutical Analytical Techniques
PQA-MQA102T: Quality Management Systems
PQA-MQA103T: Quality Control and Quality Assurance
PQA-MQA104T: Product Development and Technology Transfer
PQA-MQA105P: Pharmaceutical Quality Assurance Practical I
PQA-MQA106S: Seminar
Semester II
PQA-MQA201T: Hazards and Safety Management
PQA-MQA202T: Pharmaceutical Validation
PQA-MQA203T: Audits and Regulatory Compliance
PQA-MQA204T: Pharmaceutical Manufacturing Technology
PQA-MQA205P: Pharmaceutical Quality Assurance Practical II
PQA-MQA206S: Seminar
Semester III and IV
PHA-MRM301T: 沙巴体育 Methodology and Biostatistics
MRJ302P: Journal Club
MRW401P 沙巴体育 Work
In the Fourth semester MPharm, students choose a particular area of research and perform project work in a specified innovative area either in the college or in industry.
Why the Department of Pharmaceutical Quality Assurance?
The course has a well-structured, broad-based inclusive syllabus designed with inputs from experts and tailored to the present need. The students are trained in a GLP-compliant environment, ready to take up analytical, regulatory, and quality functions in any industry. The training and teaching are passionate with a firm belief that quality is not an accident, but the result of endless hard work. The Department is active in creating new knowledge by way of funded research*, high-impact research papers*, books, and industry collaborations*. 沙巴体育 of the department are spread across the globe and domain*. Most of them have reached the epitome of career and occupy key decision-making positions. They are in constant touch with the department contributing to the placements, knowledge pool, and development.
Thus 沙巴体育 College of Pharmaceutical Sciences is the dream destination for an aspiring graduate to obtain MPharm Pharmaceutical Quality Assurance.
-416x232.jpg)
Academics
Department offers postgraduate courses in Pharmaceutical Quality Assurance and Pharmaceutical Regulatory Affairs.
-416x232.jpg)
沙巴体育
Department provides research opportunities in the field of analytical and regulatory science through its doctoral program.
-416x232.jpg)
DADC
As a part of MCOPS Drug Analysis and Development Centre (DADC), department extends sample analysis services.
Faculty
Labs & Facilities
MU has best-in-class facilities for students of all constituent institutes
528x328.jpg)
Our lab has high end sophisticated analytical instruments like tandem LC with ion trap mass analyser, Ultracentrifuge, GCMS, DSC, HPTLC, HPLC systems with various detectors including ECD, UV, PDA and fluorescence etc.
1-304x200.jpg)
-304x200.jpg)
Licensed e-CTD Software
-359x236.jpg)
The Department has licenced e-CTD software (Take solutions) to train the students in regulatory e-filing procedures.
沙巴体育 & Publications
The department established in the year 2002 under the leadership of Dr. G. Gautham Shenoy. Since then the department has grown on its identity and reach on research. It has received grants worth few crores from various funding agencies like AICTE, DST, etc. with the liberal financial support from the university and grants from the governmental agencies, the department could establish and maintain a “state-of-the-art” sophisticated instrumentation laboratory. This is serving as a nodal analytical research Centre not only for the researchers of Pharmacy College but the whole 沙巴体育 Academy of Higher Education and neighbouring academic institutions. A large number of high impact publications have been generated by the researchers of the department using the facility. Off late, the department is active in collaborative research with various constituent institutions of the university. This collaboration has resulted in many other grants from the funding agencies like with the Department of Biochemistry from VGST, Department of Gastroenterology from DST, and Department of Medicine from ICMR, etc. The Department is also an analytical service provider and consultant for many pharmaceutical industries like Steer Engineering, Get well pharma, etc. The department also helps many students from neighbouring academic institutions like NITTE University, Yenepoya University, and SDM &Muniyal Institute of Ayurveda Medical Sciences, various pharmacy colleges across the state of Karnataka and beyond in fructifying their research efforts. Staff members of the department act as a consultant, evaluators, book authors, journal referees, editors, etc, because of their competency and contribution in the core knowledge domain.
Testimonials

“From state of the art instruments and technology to wide array of extremely talented and knowledgeable teachers, our MCOPS has it all. This combined with the guidance and support from my department really helped pave the path for my future and get me to where I am today.”
Sushant Shete
Mpharm Pharmaceutical Quality Assurance (2018 – 2020)
Senior research Associate (Trainee), Syngene 沙巴体育 Ltd.

“The college and the faculties added value to my professional and academic experience. The entire two year in MCOPS also provided a fresh approach to both research and practice in education.”
Pallavi Dhokade
M.Pharm Pharmaceutical Quality Assurance (2014 – 2016)
Regulatory Engineer, Tata Elxsi

The department & college has developed me to be better professional through rigorous teaching and interaction session and it helps me day in and day out even today.Proud to be an MCOPS alumni.
Sandeesha Lunawat
M.Pharm Pharmaceutical Regulatory Affairs (2017 – 2019)
Junior Regulatory Affairs Specialist, Covance India

“沙巴体育 university has been excellent and a memory to cherish for a lifetime. I am grateful for the effort of department of Quality Assurance to pitch us all at a platform like Novartis and other renowned companies.”
Krishnaveni
M. Pharm Pharmaceutical Regulatory Affairs (2018 – 2020)
Regulatory publishing associate, Novartis Healthcare Pvt.Ltd

“MAHE and MCOPS, its great place for student. Interacting with people all over the country in a free environment was a different experience.”
Lakshman Reddy
M. Pharm Pharmaceutical Regulatory Affairs (2018 – 2020),
Submission Delivery Specialist, GlaxoSmithKline.

“MAHE, MCOPS is platform of knowledge and technological excellence. I believe it was my best decision to join MCOPS. It’s the efforts that our faculty and college make me count myself into better professional.”
Shreya Karia
M.Pharm Pharmaceutical Quality Assurance (2018 – 2020)
Senior 沙巴体育 Associate Trainee, Syngene 沙巴体育 Ltd
%20Pharmaceutics.jpg)